Abstract
Background: Allogeneic hematopoietic stem cell transplantation (HCT) eradicates leukemic clones via intensive chemoradiation and donor-cell derived graft-versus-leukemia (GVL) effect. The pathogenesis of post-HCT relapse includes diverse mechanisms including immunophenotypic switch, immune evasion, or clonal evolution via alteration in mutational profiles. However, immunophenotypic switch post-HCT has not been well investigated as it relates to both immune escape and clonal switch.
Patients and methods: Ten-color multicolor flow cytometry (MFC) was performed at the time of diagnosis (Dx) and post-HCT relapse. Basic myeloid markers, progenitor cell markers and lymphoid lineage markers CD4/CD7 and CD56 were analyzed. Samples were processed using a Navios flow cytometer (Beckman Coulter, Miami, FL, USA). Antigen expression was determined positive if the antigen was present in more than 50% of the cells.
We analyzed immunophenotypic switch pattern from Dx to post-HCT relapse and compared it according to the cytogenetic profile at Dx, conditioning intensity and graft-versus-host disease (GVHD). Univariate and multivariate analyses were performed evaluating age, performance status, positive expressions per antigen at Dx and post-HCT relapse, conditioning regimen intensity (MAC vs RIC), occurrence of GVHD, and cytogenetic profile at initial Dx (adverse risk vs others as defined by Grimwade et al [Blood 2010]).
Results: A total of 107 relapse cases (18.0%) were identified in 593 patients that underwent HCT from October 2012 to August 2021: 72% received reduced intensity regimen (RIC). With a median 10 months’ follow-up duration, the OS rate at 12 months after post-HCT relapse was 23.1% (95% CI 14-32%) with 5.5 months of median survival (mOS) (95% CI 3.9-6.7 months).
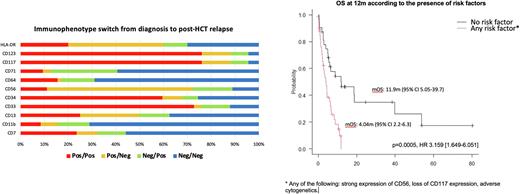
In terms of immunophenotypic switch, up to 79% of the patients showed change of antigen expression in the relapse sample compared to diagnostic sample. The rationale for the antigens chosen to study is existing research on an increasing number of them that can be targeted by immunotherapy. Of interest, 49% patients showed any changes in the potentially targetable antigens, including CD123, CD117, CD56, CD34, CD13 and CD33. Three patterns of immunophenotype switch were noted: 55% lost antigens, 53% gained antigens and 29% had concomitant gain and loss of antigens at relapse. No difference in survival was noted between those showing any immunophenotype switch vs those not (p=0.76).
In the univariate analyses, the following factors were found be adverse for OS after post HCT relapse: 1) CD56 positivity at Dx (p=0.006), 2) CD117 negativity at Dx (p=0.015), 3) CD4 positivity at Dx (p=0.002), 4) CD11b positivity at Dx (p=0.005), 5) CD71 positivity (p=0.035), 6) adverse cytogenetic risk (p=0.05): 1) CD56 positive group showed significantly shorter mOS (2.7 vs 6.5 mo); 2) CD117 negative group showed shorter mOS compared to positive group (1.9 vs 5.8 mo); 3) Adverse cytogenetic risk group showed shorter mOS compared to low/intermediate risk group (4 vs 5.1 mo). No difference of treatment modality was noted according to CD56, CD117 and cytogenetic risk groups.
In the multivariate analysis 3 risk factors were confirmed including CD56 positivity (p=0.006, HR 3.28 [1.59-6.77], p=0.001), CD117 negativity (p=0.0154, HR 2.53 [1.2-5.33], p=0.01) and adverse cytogenetic risk (p=0.05, HR 2.07 [1.1-3.86], p=0.02), while age, performance status, conditioning regimen intensity, immunophenotypic switch pattern or other antigen expression at Dx/at post-HCT relapse were not found to be significant.
A risk score model incorporating the 3 risk factors showed excellent prognostic risk stratification in post-HCT relapsed patients (p=0.0005, HR 3.159 [1.649-6.051]).
Conclusions: The prognosis of AML after experiencing relapses after HCT remains dismal with mOS of 5.5 months. Immunophenotypic switch was very common at post-HCT relapse, observed in up to 79% of patients. While CD56 strong expression and CD117 negative expression at Dx confer a worse prognosis with shorter OS after post-HCT relapse, their physiologic and biologic relevance on immune evasion needs further investigation.
Given the multitude of promising targeted therapies, repeating MFC-based antigen assessment is strongly warranted for accurate immunophenotype profiling and detection of immunophenotypic switch when considering immunotherapy as post-HCT relapse therapy.
Disclosures
Law:Kite Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Atara: Research Funding; Incyte Corporation: Research Funding; Sierra: Research Funding; Jazz Educational: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal